01
AstraZeneca local compound storage
Jack Dawson and Alex Donev
AstraZeneca, Alderley Park, Macclesfield, Cheshire, SK10 4TG, UK
Outline
Compound storage in DMSO is universally used in the department, but carries a high level of risk in terms of maintaining the integrity of the solutions for testing in biological assays. The consequences of changes to these solubilised stocks are currently poorly understood and managed. This report provides data that will clarify the key issues in this area, and give a clear way forward to avoid the associated risk.
Background
Compound storage in DMSO is universally used in the department, but carries Compounds to be tested in biological assays are dissolved in dimethyl sulfoxide (DMSO) due to its excellent solubilising ability, chemical inertness and high boiling and freezing points. However, results of studies recently published in the literature suggest that DMSO is highly hydrophilic and if left open to ambient room conditions will rapidly absorb water. This can have detrimental effects on solubilised compound stores as it may:
- Reduce stock concentration (diluted by water uptake).
- Induce compound precipitation.
- Encourage compound crystallisation in stock solutions.
- Increase compound degradation.
At the moment the storage conditions for solubilised compounds vary widely within the department and are not aligned with best practice conditions. This runs the risk of the solubilised stocks being compromised in one or all of the above ways; this can lead to errors in the stock concentration that will then propagate to all measurements where the compound is used. As a result, the concentrations of compounds used in biological assays would be lower compared to those specified by the experimental design. Consequently, inaccurate data would be used in the statistical analysis.
An experiment was carried out that would directly quantify the dilution of compounds as a result of the absorption of water by DMSO. Levels of water uptake in solubilised samples in storage conditions that are commonly used at AZ were monitored; this uptake was used to calculate the dilution of compound stocks. These data were then used to assess the impact of using inaccurate concentrations in the statistical analysis of such data, and to show how critical business decisions could be affected. a high level of risk in terms of maintaining the integrity of the solutions for testing in biological assays. The consequences of changes to these solubilised stocks are currently poorly understood and managed. This report provides data that will clarify the key issues in this area, and give a clear way forward to avoid the associated risk.
Method
This experiment used a test set of nine compounds that covered three distinct chemical series and three molecular weight bands (300, 400, 500). The compounds were solubilised in 2ml Fisher vials* with pierceable septa (air permeable) on a Tecan EVO robot, and the initial volume recorded (all volumes were calculated by weighing and using calibration curves for water and DMSO). Duplicate samples of each compound were then put in various storage conditions; room temperature, fridge (4ºC) and StoragePod (nitrogen atmosphere). The samples were weighed at regular intervals over a four-week period (typical laboratory storage period) and all measurements recorded. Figure 1 shows plots of volume gain and stock concentration against time. The typical starting volume was 500ul, so a 100ul water gain corresponds to a decrease in stock concentration from 10mM to 8.3mM.
Results
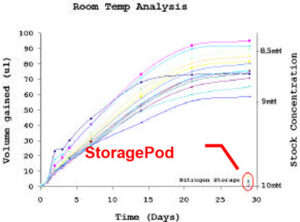
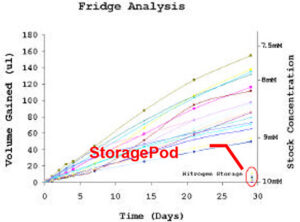
Fig 1: Plot of volume gain and stock concentration against time for 10mM sample stored in 2ml Fisher vial with pierceable septa at room temperature, in fridge and in nitrogen atmosphere. Each line corresponds to one vial of compound.
The results in Figure 1 show that the amount of water absorbed by DMSO rapidly increases with time at room temperature and at 4ºC. The compounds stored in a nitrogen atmosphere for the four-week duration showed minimal water uptake (shown on both graphs). There is considerable variability between the results when different compounds have been solubilised, particularly at 4ºC as condensation from the atmosphere occurs. Statistical analysis shows this variability does not appear to depend on chemical series or the molecular weight of the compounds, meaning the error cannot be compensated for. When the compounds were stored under nitrogen in StoragePods no problems were identified.
It is important to reiterate that the dilution of compound stocks by water is not the only issue. The effects seen below in the statistical analysis are likely to be larger due to the added risk of compound precipitation, crystallisation and degradation. During the four-week period of testing, four of the nine compounds came out of solution at room temperature and at 4ºC; again this was not an issue for the compounds stored under nitrogen.
Impact on estimation of LogIC50
Computer simulations were used to assess the downstream effect of using reduced starting concentrations. The left panel of Figure 2 shows the mean percentage changes of the estimates for the LogIC50 when the stock concentration is reduced by up to 3mM for a typical bioassay. Clearly the potency is considerably underestimated. For example, when the stock concentration has been reduced from 10 to 8mM, a compound with LogIC50 = -1.58 (IC50 = 0.0263uM, PIC50 = 7.58) would typically be found to have LogIC50 around -1.48 (IC50 = 0.0331uM, PIC50 = 7.48). As the compounds would in general be affected differently by the hydrophilic nature of the DMSO, this could affect the rank order of the compounds and lead to misleading results.
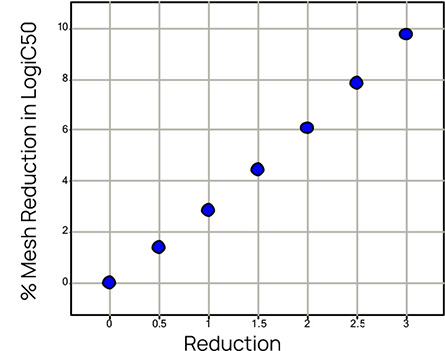
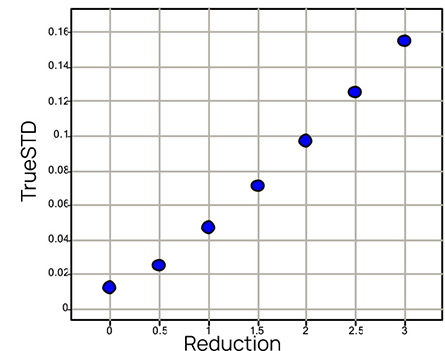
Fig 2: Mean percentage increase of the estimate of the LogIC50 and its true standard deviation for different reductions of the stock concentration.
The right panel of Figure 2 shows the mean standard deviations of the LogIC50 estimates. They are also found to rapidly increase with the reduction of the stock concentration. These differ from those that a standard statistical analysis would obtain, where the mean standard deviation would always be around that seen when the concentration is set correctly in the experiment. For example, when the stock concentration has been reduced from 10 to 8mM, the standard deviation of the estimated LogIC50 is 0.0971, rather than 0.0117 (more than eight fold larger) found in routine statistical analysis. The underestimation of the variability might lead to false perceptions of the quality of the data and to incorrect power analysis (e.g. how many observations and replications to take).
Conclusion
It has been shown that no statistical analysis would be able to correctly analyse the data because the true concentrations tested would in general be unknown. It is not possible to apply a correcting factor, as the reduction in concentration follows no discernible pattern. Therefore, it is essential to ensure that the detrimental effect of the absorption of water by DMSO is reduced as much as possible.
To this end, we have purchased equipment that will allow compounds to be stored under nitrogen and low relative humidity. These conditions are in line with best practice conditions and will ensure that solubilised compound stocks are maintained appropriately. Storing solubilised compounds in these conditions will help to minimise the risks highlighted in this document, leading to improved data quality and consequently, to better informed business decisions.
* Finneran vials (4100-1232) shell borosilicate glass clear 2.0mL 12 x 32mm.
Finneran conical snap plugs (5405SB-12) with starburst natural plug polypropylene clear 12mm.
Contact us
If you would like to discuss your system requirements with us further or have any queries, please contact us to arrange a call.